Like gravitational force that acts on all masses, the electrostatic force is another fundamental force in nature. The term ‘electric charge’ is very difficult to explain but we can describe its properties. All particles present in nature are composed of charges. The history of charges extends back to 200BC. If you want to experience charge, rub a balloon on woollen cloth and place the rubbed part against the wall. You can see the balloon stay stuck to the wall. This is because of the accumulation of static charge on the balloon.
Electric Charge
Electric charge is a character of a matter that causes it to experience a force when placed in the electromagnetic field. It is denoted by the letter q. Benjamin Franklin from his experiments in late 18th Century identified the existence of two types of charges. From his experiments he concluded the following:
- There are two types of charges- positive charge (+q) and negative charge (-q).
- Unlike charges attracts and like charges repel.
Two positive or two negative charges kept closer to each other pushes themselves apart, while a positive charge and a negative charge kept closer attracts each other. Also, it is assumed that the electric field of a positive charge always acts outwards and that of a negative charge always acts inwards as shown in the picture below:
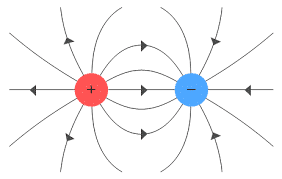
Unit of Electric Charge
Electric charge is measured in Coulombs. Coulomb is defined as the quantity of charge that passes through the cross-section of an electrical conductor carrying one ampere for one second. One coulomb is equal to the amount of charge for 6.28×1018 electrons.
If one coulomb is equal to the amount of charge for 6.28×1018 electrons, an object with one coulomb of negative charge has 6,280,000,000,000,000,000 extra electons.
Atoms are the basic part of all matters. Atoms are made up of Protons, Electrons and Neutrons. Protons and electrons are present at the centre of atom and electrons orbit them. Protons are believed to have positively charged, electrons – negatively charged and neutrons- not charged. In a balanced atom, an equal number of electrons and protons are present, making it neutrally charged. When an atom loses one or more electron it becomes positively charged and when it gains one or more electrons it becomes negatively charged. Charge possessed by an electron or a proton is approximately 1.6×10−19 C.
Transfer of charges
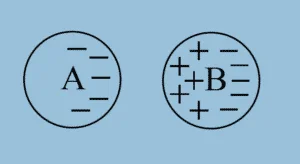
In the introductory section, you read about rubbing a balloon to the woollen cloth. By doing so electrons from woollen cloth are transferred to the balloon. This makes the balloon negatively charged. Wall is assuming to have a positive charge. Since unlike charges attract each other, the balloon stays stuck over the wall. Charges can also be transferred by induction. If a positively charged particle is brought close to a conductor, all positive charges close to the particle shall be repelled and negative charges will be attracted.
Coulomb’s Law
Coulomb’s Law states that the magnitude of electric force between two charged particles is directly proportional to the product of their charges and inversely proportional to the square of the distance between them.
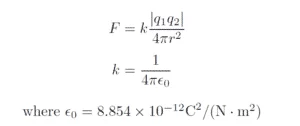
Where F is the force between the charges q1 and q2, and r is the distance between them.
Principle of Superposition of charges
Coulomb’s law applies to any pair of point charges. When more than two charged particles, the net force on any one of the charges is simply the vector sum of the forces exerted on it by the other charges. Consider if there are three charges q1, q2 and q3. The force experienced by charge q1 will be equal to the vector sum of forces exerted by q2 and q3 over q1.
Summary
All particles present in nature are composed of charges. There are two kinds of charges: Positive charge and negative charge. Unlike charges attracts each other and like charges repels each other. The unit of charge in coulombs. The amount of charge possessed by electrons is approximately 1.6×10−19 C.

“Like charges attracts and unlike charges repel” This doesn’t sound right, typo perhaps?
Thank you. Short article but highly informative